Archive for January 2014
Hello Friends, In this post we will be discussing about one of the important component used in electronics circuits. "Batteries - The Ultimate Power supply".
The main aim of this post is to let you understand the working principle behind the batteries and where/why we are going to use it in our future electronics experiments.
I will try to discuss all topics in simple way so that a new beginner can also get the point.
So, Lets start with the Batteries.
A battery contains chemicals that liberate electrons (particles of electricity), which want to flow from one terminal to the other as a result of a chemical reaction inside it.
Think of the cells inside a battery as being like two water tanks—one of them full, the other empty. If they are connected with a pipe, water flows between them until their levels are equal. Similarly, when you open up an electrical pathway between the two sides of a battery, electrons flow between them.
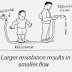
Or one can also understand the working of batteries as a tap work when we connect a water pipe to it and open its knob to get water supply from pipe. As we open its knob water flow begins- this can be thought as when we supply power with battery current flow starts in the circuit. Now if we open knob to a greater extent we see a increment in water flow- this can be thought as when we increase the value of power supply (i.e. from 6V to 9V) keeping all the circuit same our current will increase.
Now, If you’re wondering exactly how much current flows then wait we will discuss this topic in details in our later discussion.
Batteries range in size from button cells to large lead-acid units that store power generated by solar panels or windmills.
Inventor of the battery
Alessandro Volta was born in Italy in 1745, long before science was broken up into specialities. After studying chemistry (he discovered methane in 1776), he became a professor of physics and became interested in the so called galvanic response, whereby a frog’s leg will twitch in response to a jolt of static electricity.
Using a wine glass full of salt water, Volta demonstrated that the chemical reaction between two electrodes, one made of copper, the other of zinc, will generate a steady electric current. In 1800, he refined his apparatus by stacking plates of copper and zinc, separated by cardboard soaked in salt and water. This “voltaic pile” was the first electric battery.
Testing Battery Voltage
If you have a multimeter with you then it is quite easy to find out whether it is charged or not and to measure the voltage of Battery.
Procedure :
1. Connect the black-coloured test lead to the multimeter terminal (hole) marked COM .
2. Connect the red-coloured test lead to the multimeter terminal marked V.
3. We are testing for voltage; so turn the multimeter dial to the V or DCV setting.
4. Touch the metal tip of the black test lead to the battery’s negative terminal (the larger snap, which is usually marked “-”).
5. Touch the metal tip of the red test lead to the battery’s positive terminal (the smaller snap, which is usually marked “+”).
In the case you don't want to measure voltage between terminals of battery and only wants to check whether battery is charged or not you can check it simply as follows:
Moisten your tongue and touch the tip of it to the metal terminals of a 9-volt battery. The sudden sharp tingle that you feel is caused by electricity flowing from one terminal of the battery, through the moisture on and in your tongue, to the other terminal. Because the skin of your tongue is very thin (it’s actually a mucus membrane) and the nerves are close to the surface, you can feel the electricity very easily.
NOTE: No More Than 9 Volts
A 9-volt battery won’t hurt you. But do not try this experiment with a higher-voltage battery or a larger battery that can deliver more current. Also, if you have metal braces on your teeth, be very careful not to touch them with the battery.
Symbol Of battery used in circuit diagrams:
General Batteries that we will use in our experiments are:
For more stay connected and post in
comments as your reply, query and suggestions.
See you soon!!